Introduction to Medical Device Investigations
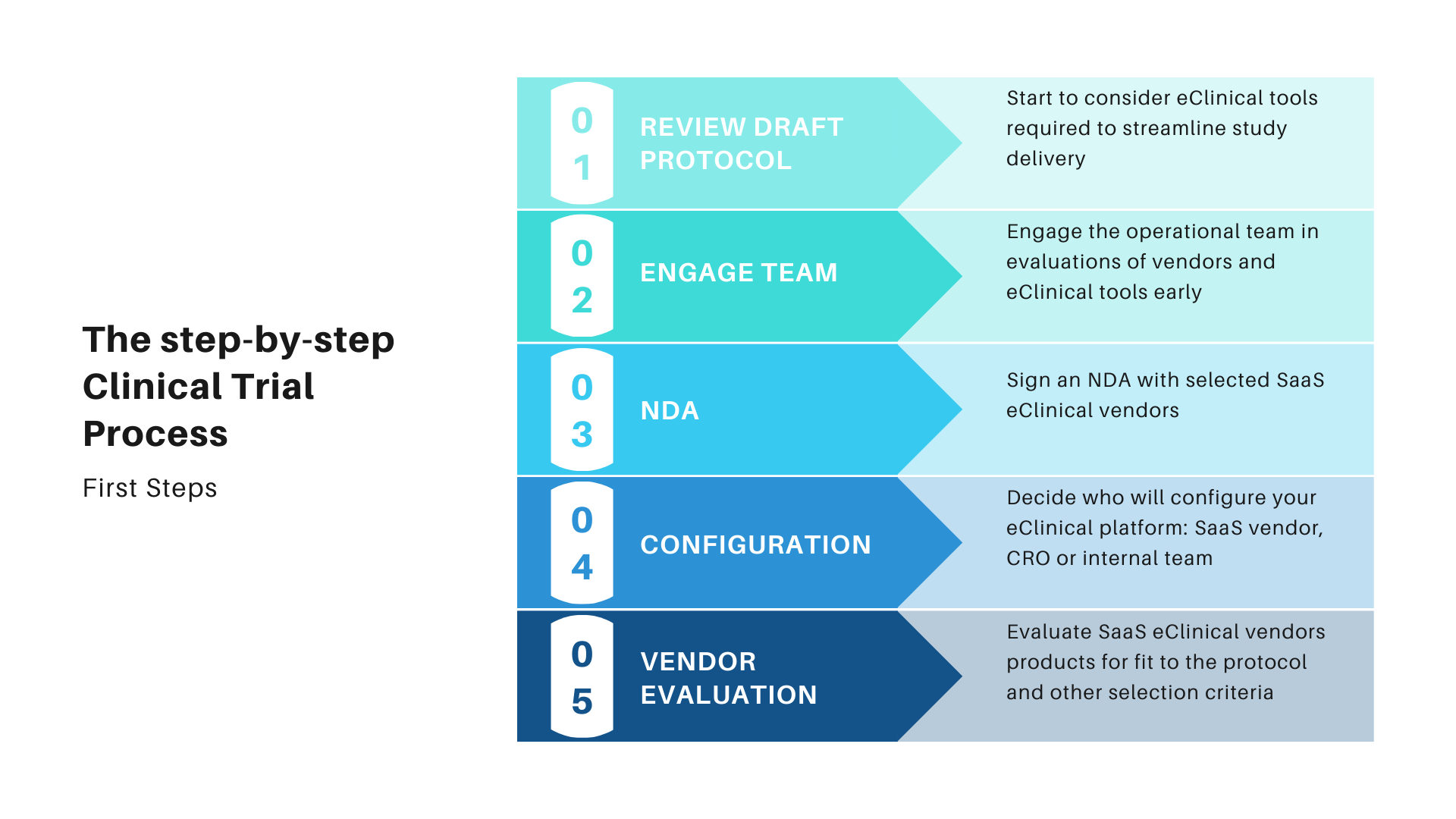
The medical device industry covers large multi-nationals with departments dedicated to R&D, compliance and clinical trial management. At the other end of the spectrum lies the SME, the smaller of which may be entrepreneurs with a great business idea and innovative product they need to get to market. Both ends of the spectrum and all those in between have the same basic regulatory requirements and processes to fulfill. Our objective here is to help give some basic guidance to those for whom clinical investigations are maybe a one-time challenge, not a career. If that sounds like you, you’re in the right place!
The section contains links to our ‘info sheet’ series, relevant articles and our medical device study glossary.