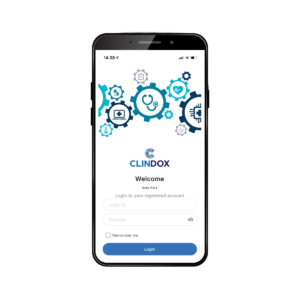
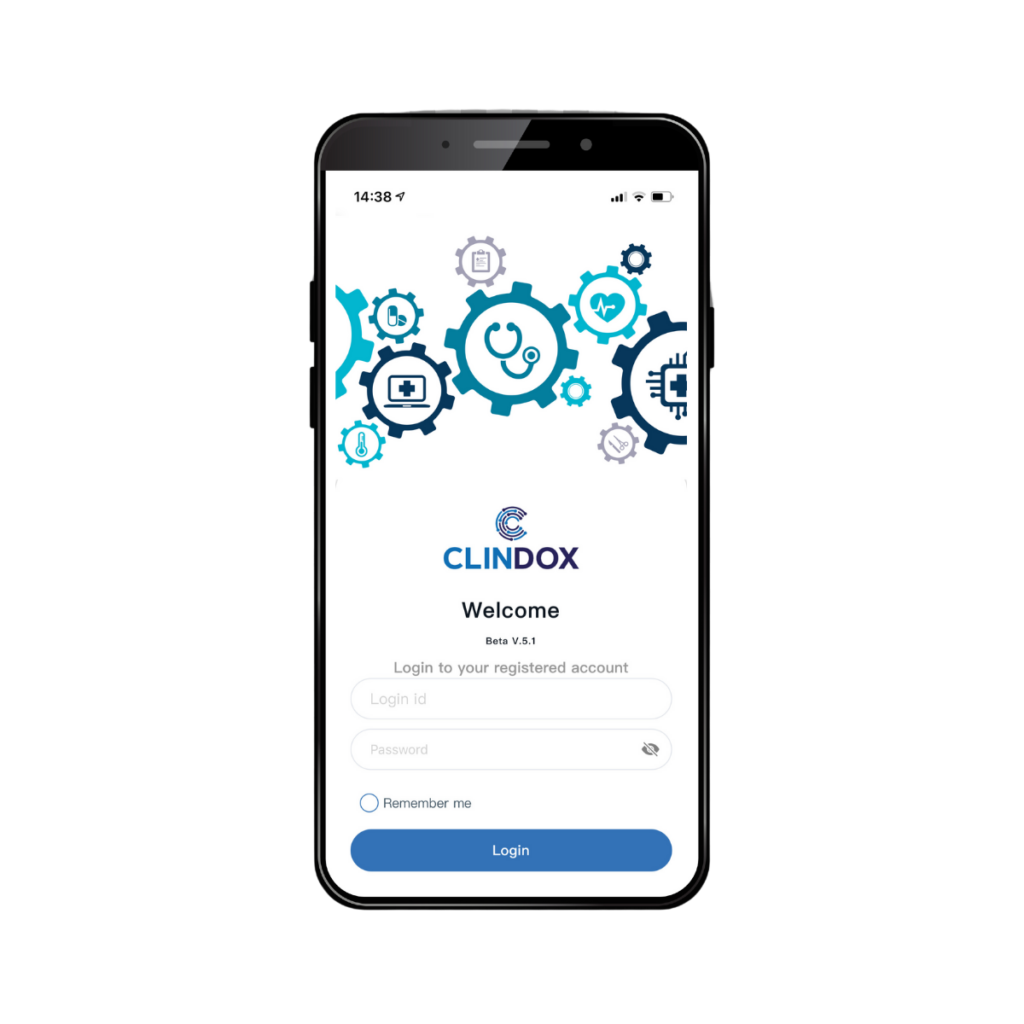
Like all features of CRFWEB, the eDiary function is fully integrated with the application, providing a simple, user-friendly access to our ePRO functionality. eDiary is available to subjects via login to a mobile device for daily observations and has the added benefit of improving data collection responses through reminders and alerts. eDiary can be accessed using a supplied, fully provisioned device or via the subject’s own mobile device via the Clindox App.
Europe (UK): +44 1732 316319 | India: +91 20 71531039 | USA: +1 855 2596535


eDiary
Core eDiary functionality
- Prepare a schedule / calendar for data capture and assign subjects intended to be part of the study
- Login through Android / iOS devices to record daily observations
- Complex formulas and edit checks to manage the study complexity
- Notification to investigators/Nurses to visit Subject on any observations during data capture
- No risk of subject accessing other functionality or data
- Notification/Alerts to Subject for any data which needs quick action by Subject eg Visit to Doctor, Nurse, etc
View the eDiary subject user experience
through the Clindox App
5 Star Reviewed
5 Star rated on Capterra
What our clients say
" The Clindox team have been an incredible support and are always available to help in any way they can."
" The time and cost saving is really significant. We're very happy with the performance or CRFweb and I wouldn't hesitate to recommend it."
" CRFWEB eCRF, plus their helpful and accommodating staff, helped us quickly set up and personalize, with quick turn-around times of modifications, exactly what we, the clinical staff, and statistical consultants demanded, all at an affordable price "
" The Clindox (CRFWEB) team are dedicated and enthusiastic about accommodating the unique requirements of each individual study and are great value for money."
"Our international CRO business has utilized Clindox's integrated randomization module and Kit Management. The functionality provides everything we need, and having key trial components integrated into one system is a huge advantage to us in terms of trial efficiencies. Having both randomization and kit management capabilities, aligned with the EDC really simplifies the whole process and significantly reduces the time and effort required to manage our studies and keep control of our processes and inventory. "
Next Steps
For a quick system overview, click here
Seeing is believing. Book a web-based demo now.
Useful Links
Latest News
Covid-19: What has been the impact of the pandemic on India and how have Indian Clinical Trials been affected?
Decentralized Clinical Trials – new kid on the block or a venerable idea resurrected?
Registered number: 526690 © Copyright CRFweb 2019