Europe (UK): +44 1732 316319 | India: +91 20 71531039 | USA: +1 855 2596535
 Introducing the all new Clindox App - state-of-the-art direct data capture in your subjects hands. Learn more here...
Introducing the all new Clindox App - state-of-the-art direct data capture in your subjects hands. Learn more here...
 Introducing the all new Clindox App - state-of-the-art direct data capture in your subjects hands. Learn more here...
Introducing the all new Clindox App - state-of-the-art direct data capture in your subjects hands. Learn more here...
Your clinical data technology partner …together we create better studies
We provide state of the art software and service solutions that help you to collect, manage and analyse clinical data more efficiently to support regulatory compliance and evaluate scientific, technical, and operational innovations. We do this with a human touch. We’ll work with you to make your next First-in-Human, Pivotal, PMCF, PMSS, Registry or IIS study the best one yet.
The Clindox Difference
Reduce your time to market
Study set-up in a matter of days. We will set-up for you, or you can build your own.
EDC or ePRO/eDiary – data capture how you need it
Investigator or subject led data collection on any device. All the features you need in an intuitive and integrated application.
Market proven, secure and compliant
Meet your regulatory and business objectives.
Outstanding Customer Service
A trained, experienced human being will respond to your query and prioritise it’s resolution.
CRFWEB …everything you need in one integrated application.
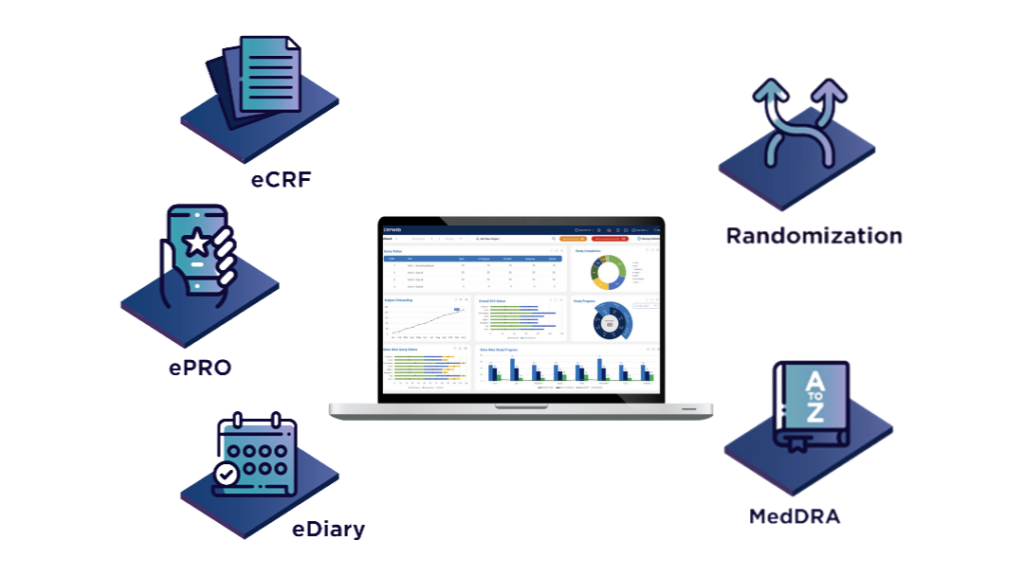
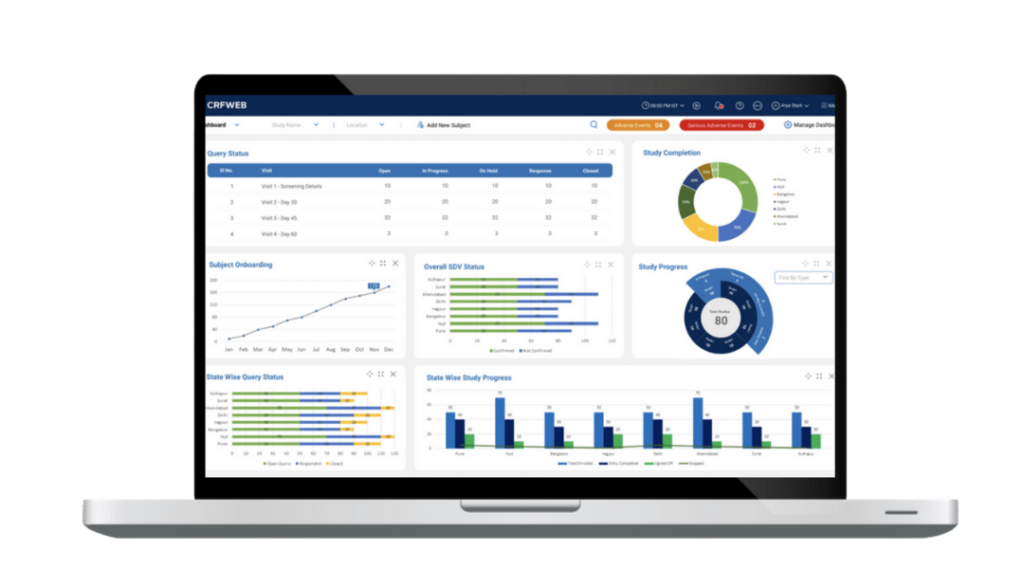
To see how it all works click here
Seeing is believing. Book a web-based demo now.
What Our Clients Say

" The Clindox team have been an incredible support and are always available to help in any way they can."
" The time and cost saving is really significant. We're very happy with the performance or CRFweb and I wouldn't hesitate to recommend it."
" CRFWEB eCRF, plus their helpful and accommodating staff, helped us quickly set up and personalize, with quick turn-around times of modifications, exactly what we, the clinical staff, and statistical consultants demanded, all at an affordable price "
" The Clindox (CRFWEB) team are dedicated and enthusiastic about accommodating the unique requirements of each individual study and are great value for money."
Products and Features

eCRF

ePRO
eDiary
Medical Device Investigations

Market-tested. Proven. Cost-effective EDC.
Currently helping medical device companies meet their business and compliance requirements.
Latest News
Covid-19: What has been the impact of the pandemic on India and how have Indian Clinical Trials been affected?
Decentralized Clinical Trials – new kid on the block or a venerable idea resurrected?
Registered number: 526690 © Copyright CRFweb 2019